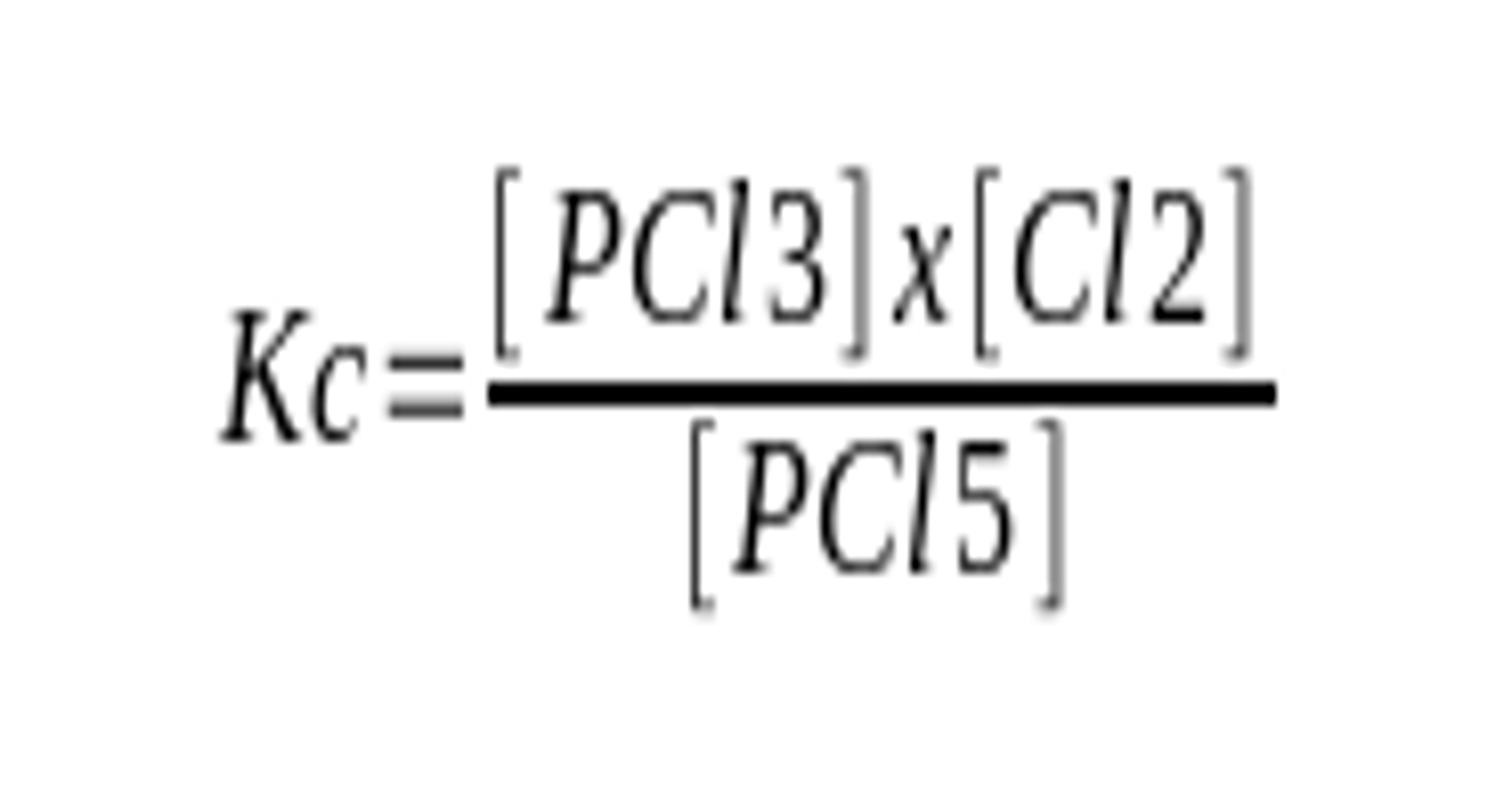
Write down the equilibrium law (K) formulas for the following reactions.
Contents
Question
A. $ \ce{PCl5(g) ⇄ PCl3(g) + Cl2(g)} $
B. $ \ce{2SO3(g) + 2Cl2(g) ⇄ 2SO2Cl2(g) + O2(g)} $
C. $ \ce{CO(g) + 2H2(g) ⇄ CH3OH(g)} $
D. $ \ce{Cu^2+(aq) + Zn(s) ⇄ Zn^2+ (aq) + Cu(s)} $
E. $ \ce{2NH3(g) + CO2(g) ⇄ NH2CO2NH4(s)} $
Note
Write only the formulas with fase g and aq, and the formula is the reactants products by divide or the right side divided by the left side. And the constant is the exponent.
Answer
A. $ \ce{Kc = [PCl3] \times [Cl2] / [PCl5]} $
B. $ \ce{Kc = [SO2Cl2]^2 \times [O2] / [SO3]^2 \times [Cl2]^2} $
C. $ \ce{Kc = [CH3OH] / [CO] \times [H2]^2} $
D. $ \ce{Kc = [Zn^2+] / [Cu^2+]} $
Exercise
E. For this problem, you work on it yourself 😄
Information
The next update will be available in Sundanese.
content copywrite


